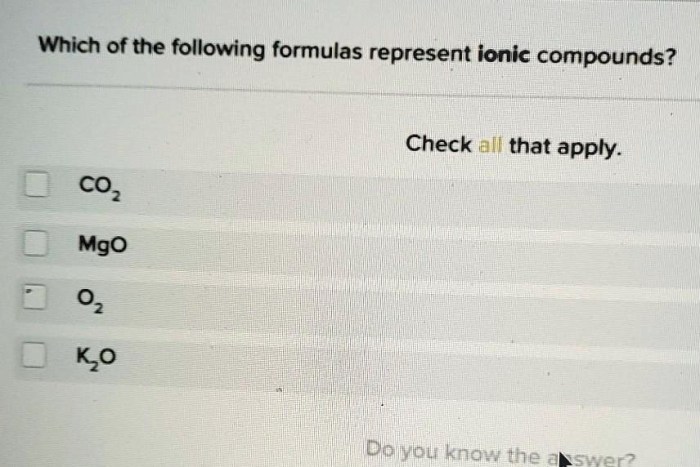
Which of the following formulas represent ionic compounds – Delving into the realm of ionic compounds, this exploration unveils the intriguing world of chemical bonding and formula representation. Ionic compounds, characterized by their unique properties and ubiquitous presence, play a pivotal role in various scientific disciplines and everyday applications.
Through a comprehensive examination of their characteristics, bonding mechanisms, and formulaic intricacies, this discourse provides a foundational understanding of ionic compounds, empowering readers to decipher their chemical identities and unravel their significance in the grand tapestry of chemistry.
Ionic Compounds: Which Of The Following Formulas Represent Ionic Compounds
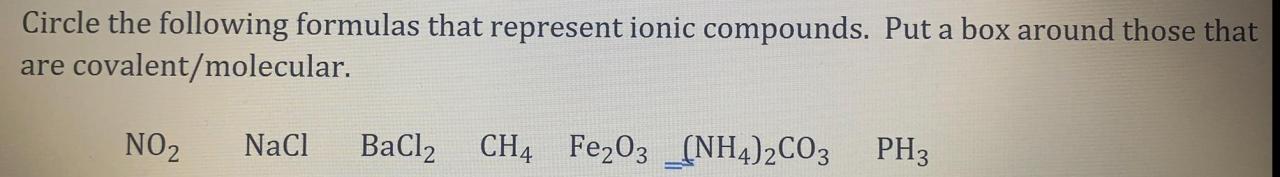
Ionic compounds are chemical compounds composed of ions, which are atoms or molecules that have lost or gained electrons. They are formed when a metal loses one or more electrons to a nonmetal. The resulting ions are attracted to each other by electrostatic forces, forming an ionic bond.Ionic
compounds are typically hard, brittle, and have high melting and boiling points. They are also good conductors of electricity when dissolved in water or melted.
Formula Representation of Ionic Compounds
Ionic compounds are represented by chemical formulas that show the ratio of the ions in the compound. The formula unit of an ionic compound is the simplest whole-number ratio of the ions in the compound. For example, the formula unit of sodium chloride (NaCl) is 1:1, meaning that there is one sodium ion for every chloride ion in the compound.
Distinguishing Ionic Compounds from Other Compounds, Which of the following formulas represent ionic compounds
Ionic compounds are different from covalent compounds, which are formed when two nonmetals share electrons. Ionic compounds are typically more stable than covalent compounds and have higher melting and boiling points. They are also better conductors of electricity.
Chemical Equations Involving Ionic Compounds
Ionic compounds can participate in chemical reactions, such as precipitation reactions and acid-base reactions. In a precipitation reaction, two ionic compounds react to form an insoluble solid. In an acid-base reaction, an acid and a base react to form a salt and water.
Applications of Ionic Compounds
Ionic compounds have a wide range of applications, including:
- As table salt (NaCl)
- As fertilizers (NH4NO3, KNO3)
- As cleaning agents (NaHCO3, Na2CO3)
- As medicines (LiCl, CaCO3)
- As industrial chemicals (NaOH, HCl)
Essential Questionnaire
What are the key characteristics of ionic compounds?
Ionic compounds are characterized by their high melting and boiling points, solubility in water, and ability to conduct electricity when dissolved or molten.
How do ionic bonds form?
Ionic bonds result from the electrostatic attraction between positively charged cations and negatively charged anions, formed when atoms transfer electrons to achieve stable electron configurations.
What is the significance of formula units in ionic compounds?
Formula units represent the simplest whole-number ratio of ions in an ionic compound, providing insights into the compound’s stoichiometry and charge balance.